A breakthrough in Cancer Treatment or Cancer cell
proliferation is inhibited by specific modulation frequencies but how and
why?? Some new findings by Dr
Chris Barnes, Bangor Scientific and Educational Consultants, Wales, UK. and a comprehensive but totally independent
explanation of the new cancer treatment
device used by Zimmerman et al (2013) see https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3845545/
and https://www.therabionic.com/
Dr Barnes' EMAIL manager@bsec-wales.co.uk. First published online2018, re-published online 11/11/2022
Abstract
The work of Zimmerman et al (2013) is first
elucidated in the context of the history
of research into the biological effects of electromagnetic fields (EMFs). Dubost’s theory of microtubule resonance has been considered
by some to account for the effects of TTF (tumour treating frequencies) and
although it could be modified to incorporate solitonic ( hence higher Q)
behaviour it does not immediately elicit
a reason or reasons why Zimmerman observed these treatment frequencies to bring about immediate physiological changes
in blood pressure and heart rate, see https://www.therabionic.com/therapy-with-the-therabionic-p1-medical-device/#mechanismoffrequencies. Amongst the questions I answer are:
1. Is the
effect of Zimmerman fundamentally different from that of Kirson?
2. What
is the mechanism of the effect of Zimmerman -they know they produce multiple
effects, but they state they do not know how?
3. If ion channels are involved, how ?
4. How
can the downregulation of both XCL2 and PLP2 be explained?
In answer it is proposed that a variant of Ion Cyclotron
resonance accounts for the precise TTF frequencies involved based on allowing
for very high harmonic frequencies to develop
upon dehydration due to lowered dielectric constant and conservation of
angular momentum within ion channels, the model is developed and tested. The importance of GMF (local geomagnetic
field) is emphasised. The system is
shown to be different from that of
Kirson.
The observed down regulation of XCL2 is accounted for in
a putative link between inflammation,
invasion and metastasis and intra-cellular Ca2+ ion concentration has been
proposed wherein it seems the
TTF modulated signal is acting rather like the chemotherapy drug
Infliximab and in this case causing Ca2+ efflux or slowing Ca2+ entry,
hence downregulating XCL2. Either way
the action, brought about by the tumour treating frequencies modulated onto the
27.12 MHz signal is deep within the hydrophobic part of the channel as
identified by the high ICR harmonics present.
A distinct breakpoint in the spread of the ICR Harmonics
for calcium across the TTF frequency spectrum is observed. This is entirely consistent with the
progressive and known and literature documented dehydration of calcium as it
passes through the pore and is also entirely consistent with a combination of
both the dielectric and angular momentum models which I propose.
TTF’s also effectively suppress K+ current giving the
link for reduced PLP2 and another explanation of the observed damage to
the mitotic spindle. Unlike previous
authors which only focus on electromagnetic interaction with calcium ions as a
second messenger, it is further shown that all sorts of ion channels and
transporters can interact through ICR with their ions and/or ligands.
Overall, simple sequential plots of closest to integer
ICR harmonic numbers obtained from both the HCC and the Breast Cancer Treatment
file yield ‘channelopathy’ like results showing break-points at places which
can be interpreted as corresponding to those of known dehydration such as the
narrow pore and selective filter.
The ‘channelopathy’ hypothesis is especially reinforced
by considering the result for voltage gated proton channels where as expected
the plots are essential linear and have no sharp breakpoints.
Introduction
Cancer is one of the
major diseases of the 21st Century.
To identify drug free modalities for treatment which offer patient
convenience, significantly less patient trauma and a link with far better cost
benefit analysis seems like a pipe-dream.
Nevertheless, there is a new system has been trialled in Brazil for HCC
(liver) and Breast Cancer which appears to do just that. Zimmerman et
al British Journal of Cancer
volume 106, pages 307–313 (17 January 2012) describe how cancer cell
proliferation is inhibited by specific modulation frequencies. The Zimmerman et al (2012) study with the
group’s two preceding papers (Barbault et al, 2009; Costa et al, 2011),
identify such a modality. Their set of clinical and explanatory laboratory
results which achieve outcomes in metastatic patient prognoses as good as
modern chemotherapy regimens and downregulation of two specific genes XCL2 and
PLP2 remains to be explained. Indeed,
the researchers themselves state that there is presently no known physical
mechanism for these effects or their method of therapeutic action. It is my present intention to elucidate
precisely such mechanism and to make this work freely available on my website
in the interests of open innovation and to the benefit of human kind.
It is thus my
present contention that we need to first understand this work in the context of
the history of research into the biological effects of electromagnetic fields
(EMFs).
The beginning of
the 20th century
saw the first
medical applications of electromagnetic fields (EMF), notably in the
diagnosis and therapy of
various diseases such
as cancer. The
assumption was that external application of electromagnetic energy could
correct disease-causing altered electromagnetic frequencies or energy fields
within the body. Abrams (ref)
invented various machines
with the goal
to cure cancer. However, between 1923 and 1924, Scientific
American magazine set up a committee
to investigate Abrams’s results
and concluded “the claims advanced on behalf of the electronic reactions
of Abrams, and electronic practice in general, are not substantiated”
Lakhovsky developed
the Radio-Cellulo-Oscillator in the 1920s this device produced broad band high frequency (RF) EMF around 150
MHz. He postulated that EMF reinforced “the
oscillations of the cell.” Although a
controversial figure in his time,
he seems to
have had some success with his
treatments (refs).
Raymond Royal
Rife hypothesized that a number of bacilli were causal factors in
many diseases, especially
cancer. In the
mid 1930s, he
developed a microscope able to see these
bacilli and invented
the Rife Frequency Generator, commonly called Rife Ray
Machine, which he claimed could diagnose
and eliminate diseases like cancer by tuning
into electrical impulses
given off by
diseased tissue. Rife’s machine produced some 400 watts of
output power fed into a gas discharge tube antenna. This would have radiated a lot of soft
x-rays and ultraviolet light and being very inefficient as a radio antenna,
only about 1 watt of RF. The former,
especially the u/v could have
instrumental in killing bacteria.
Although there were anecdotal reports that he had cured someone’s
cancer, the American Medical Association later condemned Rife’s experiment.
Until very
recently, virtually all
medical devices aimed
at treating cancer using
low levels of
electric and/or magnetic
fields were considered nothing
more than quackery because of lack of scientific proof.
Yet EMF has, for some time, also been used as a therapeutic modality for
osteoarthritis. Alternating electric
fields have been
used to induce
fracture healing, with
suggested efficacy similar
to that of
bone graft. The
proposed action of pulsed EMF in
this case is through the induction of directed migration and differentiation of
bone marrow-derived mesenchymal
stem cells.. Currently, RF EMF is used as a therapeutic option in cases
ranging from tibial stress fractures to spinal cord injury. Clearly, such successes are evidence of the
start of a paradigm change.
High power
Radiofrequency ablation (RFA) is a therapeutic option sometimes used
to treat malignancies
including breast cancer,
colorectal cancer, and hepatocellular carcinoma (HCC), and
especially surgically unresectable
metastases. RFA has been
administered with medical
devices operating at numerous different frequencies including
circa 500KHz, 2.2 MHz, 13.56 MHz, 27.12 MHz and 915 MHz and
delivering therapeutic
energy to soft
tissues. This modality primarily destroys
tumor tissue through
heat-induced necrosis by
raising their temperature
to greater than 45 C and often to
approximately 100°C for approximately 15 min.
There is a growing
body of laboratory and clinical evidence suggests that certain frequencies
within the RF EMF range of the spectrum may have antitumor effects without
eliciting temperature increase. Such
effects are often described as non -thermal or subtle field effects of EMF and
RF radiation. Likewise, there is a
huge body of evidence that considers the potential dangers of such radiation to
biological systems and life in general.
Kirson et al (2004)
employed low-intensity, intermediate-frequency (100–300 kHz), alternating
electric fields, delivered by means of insulated electrodes, and found them to
have a profound inhibitory effect on the growth rate of a variety of human and
rodent tumor cell lines. This effect was
shown to be nonthermal and to selectively affect dividing cells while quiescent
cells are left intact. These fields acted
in two modes: arrest of cell proliferation and destruction of cells
while undergoing division. Both effects were
demonstrated when such fields are applied for 24 h to cells undergoing
mitosis that is oriented roughly along the field direction. The first mode of
action is manifested by interference with the proper formation of the mitotic
spindle, whereas the second results in rapid disintegration of the dividing
cells. Both effects, which were
frequency dependent, were shown by the authors to be consistent with the computed
directional forces exerted by these specific fields on charges and dipoles
within the dividing cells. In vivo treatment of tumors in C57BL/6 and BALB/c
mice (B16F1 and CT-26 syngeneic tumor models, respectively), resulted in
significant slowing of tumor growth and extensive destruction of tumor cells
within 3–6 days. These findings demonstrated the potential applicability of the
described electric fields as a novel therapeutic modality for malignant tumors.
In a second and
later study (2007), Kirson dealt with ‘Alternating electric fields
arrest cell proliferation in animal tumor models and human brain tumors’. Their findings on mice
led to the initiation of a pilot clinical trial of the effects of
TTFields in 10 patients with recurrent glioblastoma (GBM). Median time to
disease progression in these patients was 26.1 weeks and median overall
survival was 62.2 weeks. The time to disease progression and OS values are more
than double the reported medians of historical control patients. No
device-related serious adverse events were seen after >70 months of
cumulative treatment in all of the patients. The only device-related side
effect seen was a mild to moderate contact dermatitis beneath the field
delivering electrodes. These were impressive results and they concluded that
TTFields are a safe and effective new treatment modality which effectively
slows down tumor growth in vitro, in vivo and, as demonstrated in human cancer
patients.
Dubost et al (2014)
have discussed TTF in terms of microtubule mechanical resonance.
The polar and kinetochore microtubules of those cancer cells are 5 to 9 μm
long, which provides good correlation between theory and experimental results
using PEFT frequencies of 100 kHz to 200 kHz, i.e. those of Kirson. Dubost also discusses the recent work of
Zimmerman et al (refs) where they
proposed that cancer cell
inhibition in this case is caused by
PEFT applied to the straight astral microtubules at their longitudinal
mechanical resonance frequencies.
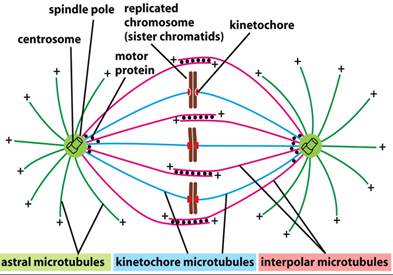
In my opinion, the
paper of Dubost is in fact very general and does not account for the manifold
yet highly precise combinations of frequencies observed by Zimmerman and Pasche
nor does it account for the precise genetic effects observed. Others have noted that microtubules (MTS) and
the spaces between act like highly non-linear ionic wires (refs) and I
have considered that it may be possible for there to be soliton modes at play
here analogous to those proposed by Geesink and Meijner(ref). Pang et al ( 2016) have experimentally
confirmed the existence of soliton modes in collagen in the infra-red and shown a
change of binding energy with applied E -field although of course this is a very different frequency range from that
considered here.
Priel et al (2006) propose a new signalling mechanism in the
cell, especially in neurons, that involves clouds of ions surrounding protein
filaments which may travel without significant decay along the axon or the dendritic
tree. Further they state that these signals could be utilized to control various membrane
properties, for example, the transition rate of ion channel opening and
closing, local membrane conductivity, and vesicle trafficking.
Dubost’s theory does not immediately elicit a reason or
reasons why the treatment frequencies
bring about immediate physiological changes in blood pressure and heart
rate, see https://www.therabionic.com/therapy-with-the-therabionic-p1-medical-device/#mechanismoffrequencies.
I propose that ion channels must be involved. Potentially Priel holds the link but I intend
to explore that in much more significant detail in a future publication.
Clearly thus there
are questions which need urgently to be
answered in order to bolster credibility for this potentially, crucially
important treatment technique.
The work of
Zimmerman et al differs from that of Kirson in that the fundamental applied
radio carrier frequency is some two
orders of magnitude or so higher and in that the former also
applies a range of complex and very
specific modulation frequencies. The
frequencies were first discovered by Barbault et al ( 2009). The questions I shall attempt to answers are as follows:
- Is the effect of
Zimmerman fundamentally different from that of Kirson?
- What is the
mechanism of the effect of Zimmerman -they know they produce multiple effects,
but they state they do not know how?
- If ion channels are involved, how ?
- How can the downregulation of both XCL2 and PLP2 be explained?
- Is there a way other than the hypothesis of Dubost for explaining
the mitotic spindle effects?
At first sight
the Zimmerman et al rationale for use of AM
modulated 27.12 MHz frequencies for the treatment of cancer seems a
little odd. It was based on previously identified several frequencies in
patients with chronic insomnia using
biofeedback methods. They had demonstrated that the intrabuccal
administration of very
low and safe
levels of 27.12 MHZ
( 100mW) MHz RF EMF, amplitude-modulated at 42.7 Hz, has a
sleep-inducing effect in
healthy subjects. However, administration of the
same signal to patients with
insomnia did not yield any therapeutic benefits. In contrast,
administration of a
combination of the
four frequencies most commonly
identified in patients with chronic insomnia (2.7 Hz, 21.9 Hz, 42.7 Hz, and 48.9 Hz) resulted in
significant improvements of total sleep
time and sleep latency as assessed by polysomnographic evaluation. It is interesting to note that these
modulation frequencies are very similar to those employed in biological
experiments which have showed frequency, field, and power windowing effects in
response to the interaction of either
ELF or modulated RF with tissue ( refs) and which have been interpreted in
terms of the theory of Ion Cyclotron Resonance (ICR) or theoretical derivatives thereof
(refs). Taking into account that
these experiments were performed in
Brazil where the earth’s geomagnetic field is of the order of 26 micro-Tesla,
it is my view that the frequency of 2.7
Hz is especially poignant. It calculates to be exactly the ICR frequency for
the amino acid L-tryptophan the most
essential for sleep especially in subjects with insomnia or increased
sleep latency, see Hartmann (1982).
They then go on to
state that the frequencies discovered with cancer patients were not the same as
those effective against insomnia.
Further they state that the frequencies were postulated/discovered by
Barbualt in an earlier study which they
reference as 2001. Every search on the
criteria given yield the paper of Barbualt et al (2009). https://www.researchgate.net/publication/24277597_Barbault_A_Costa_F_Bottger_B_Munden_R_Bomholt_F_Kuster_N_Pasche_BAmplitude-modulated_electromagnetic_fields_for_the_treatment_of_cancer_discovery_of_tumor-specific_frequencies_and_assessment_of_a_nove
Their stated rationale was because in vitro
studies suggest that low levels of electromagnetic fields may modify cancer
cell growth, they hypothesized that systemic delivery of a combination of
tumor-specific frequencies may have a therapeutic effect.
They undertook a study to identify tumor-specific
frequencies and test the feasibility of administering such frequencies to
patients with advanced cancer. We examined patients with various types of
cancer using a noninvasive biofeedback method to identify tumor-specific
frequencies. This involved monitoring
heart rate and also patient reporting changes in heart rate! They
offered compassionate treatment to some patients with advanced cancer
and limited therapeutic options. They
examined a total of 163 patients with a diagnosis of cancer and identified a
total of 1524 frequencies ranging from 0.1 Hz to 114 kHz. The specific frequencies themselves are not
all disclosed. However, all three
studies in the Pasche, Zimmerman , Barbault group appear to disclose some
frequencies which are common to all the cancers they evaluated.
These are
inter-alia 2,221.323 Hz TTF, 6530.24
Hz TTF and 10,454.4 Hz TTF.
They claim most frequencies (57-92%) were
specific for a single tumor type. The large range in brackets is not
examined. Compassionate treatment
with tumor-specific frequencies was offered to 28 patients. Three patients
experienced grade 1 fatigue during or immediately after treatment. There were
no NCI grade 2, 3 or 4 toxicities. Thirteen patients were evaluable for
response.
Clearly there were
some interesting results. One patient
with hormone-refractory breast cancer metastatic to the adrenal gland and bones
had a complete response lasting 11 months. One patient with hormone-refractory
breast cancer metastatic to liver and bones had a partial response lasting 13.5
months. Four patients had stable disease lasting for +34.1 months (thyroid cancer
metastatic to lung), 5.1 months (non-small cell lung cancer), 4.1 months
(pancreatic cancer metastatic to liver) and 4.0 months (leiomyosarcoma
metastatic to liver). In essence, their
results are comparable with those expected of chemotherapy.
I have made an earlier and somewhat simplified
attempt to explain these effects ( ref) however, since that explanation new
information has come to light which enables a far more comprehensive
explanation.
What are Zimmerman’s other frequencies?
Besides using the
downloadable files, there are three of which,
described as; Breast tumour treating frequencies, HCC (liver) treating
tumour frequencies and Random frequencies which did not provoke effect, I have also
found it instructive to consult the patent literature. BRPI0810084 (A2) ― 2014-03-18
discloses an ‘Electronic system for
influencing cellular functions in a warm-blooded mammalian subject’. Claim
20 discloses A system (11)
according to any one of the preceding claims, in which the control information
is selected to lead the one or more generator circuits (29) to generate one or
more amplitude- modulated low energy electromagnetic emissions, the frequency
of which amplitude modulations being controlled by the one or more frequency
control generators (31) at frequencies selected from at least one frequency
within the range of at least one of the following frequency ranges: 1870 to
1876 Hz, 2218 to 2224 Hz, 3666 to 3672 Hz, 4483 to 4489 Hz, 5879 to 5885 Hz,
6347 to 6353 Hz, 8459 to 8455 Hz, 10453 to 10459 Hz, a combination of two or
more frequencies within two or more of said frequency ranges, and a combination
of at least one of said frequencies and at least one further determined or
predetermined frequency outside of said frequency ranges, and
Claim 21 discloses a
system (11) according to claim 20, in which the frequencies are selected from
at least one of the following frequencies: 1873.477 Hz, 2221.323 Hz, 3669.513
Hz, 4486.384 Hz, 5882.292 Hz, 6350.333 Hz, 8452.119 Hz, 10456.383 Hz, a
combination of two or more of said frequencies, and a combination of at least
one of said frequencies and at least one further determined or predetermined
frequency.
Rightly or wrongly,
I assume that these are the groups of frequencies common to all cancer patients
and not the undisclosed remainder of the aforesaid 1524 frequencies.
I have previously
postulated that the frequencies have at least in some way a relationship with
ion channels or the perturbation thereof,
as stimulation of such channels
in excitable tissue is the only way to ever elicit the observed
biofeedback response. Excitable
tissue is defined as being nerve and muscle tissue. There is now considerable evidence that the
same types of ion channel that are found in excitable tissue are also expressed
in tumour tissue but not in healthy non-excitable tissue. This would then elegantly account for the
reason these types of treatment have no adverse effect on healthy tissue. Previously it was thought there was only a
handful of ion channels for the main biological ions. However, there is now a significant and
growing evidence for a huge number of families and genetic variations in all
kinds of ions channels included voltage gated channels, dual pore channels,
aquaporins and piezo channels to name but a few. This fits elegantly with the observation
of 1524 different treatment frequencies.
I postulate there will be a least one per different ion channel and some
for special combinations of channels and dual pore channels and the like. On the other hand Schönherr (2005)
suggests that although the current pattern of cancer-related ion
channels is not arbitrary yet it can be reduced to few members from each ion
channel family. Thus I postulate this probably accounts for the
observation of the common frequencies
mentioned above.
How can modulated RF radiation influence ion channels
anyway?
Galvanovskis and
Sandblom (1197) showed that even very weak low-frequency electromagnetic
signals (<100 Hz and down to 100 microT) may be detected in a cellular
system with a large number of ion channels.
But what is the evidence for the detection of such systems with higher
frequencies or modulated signals?
Bawin and her
coworkers have reported changes in binding of calcium after exposure of avian
brain tissue to nonionizing electromagnetic radiation. Blackman et al ( 1979) used the forebrains
of newly hatched chickens, separated at the midline to provide
treatment‐control pairs and
labelled them in vitro with radioactive calcium. Samples of tissue were exposed
for 20 minutes in a Crawford irradiation chamber to 147‐MHz radiation,
which was amplitude modulated sinusoidally at selected frequencies between 3
and 30 Hz. Power densities of incident radiation ranged between 0.5 and 2 mW
cm−2. Compared with nonirradiated samples, a statistically significant
increase in efflux of calcium ions (P < 0.01) was observed in irradiated
samples at a modulation frequency of 16 Hz and at a power density of 0.75 mW
cm−2. Their data confirmed the
existence of the frequency “window” reported by Bawin et al., as well as a
narrow power‐density “window” within which efflux of calcium ions is
enhanced. Such frequency windows can be interpreted in terms of Ion Cyclotron
Resonance (ICR).
Habash,
Electromagnetic Fields and Radiation: Human Bioeffects and Safety (2018) ,
describes numerous other examples.
Ramachandran (
2007) has shown experimentally that voltage gated ion channels are
capable of responding to an 800 MHz RF carrier wave effectively by a process of
rectification due to the combination of
membrane capacitance and non-linearity in the channel itself.
D'InzeoStefano et al
(1993) has discussed a stochastic model
of Ionic channel gating under electromagnetic exposure. They considered the membrane channel as a
non-deterministic state machine. Its behaviour is fully described by a set of
states, a matrix of transition rates, and a vector for the probability of the
machine to be in each single state at a certain instant. A stochastic model was developed, generating
random processes where the probability for each state is an aleatory variable.
The model was tested for both voltage gated and ligand-dependent channels, both
unexposed and exposed to EM fields in the ELF range.
Intuitively, I would
propose that rectification or demodulation of a modulated HF carrier wave would
occur at cell membranes and hence expose ion channels to the modulation
envelope frequency component. Indeed, there is evidence to support my
claim. Elnasharty et al discuss ‘cell membrane
analysis using modulated electrophoresis.
They describe method of examining this low-frequency region using a low
frequency signal to modulate a 1 MHz carrier wave, allowing membrane
conductance due to conduction through ion channels and surface conductance of
the membrane to be probed in this unusual way for the first time. They produce
DEP spectra before and after the application of ion channel blockers.
The technique works
because demodulation of the AM signal occurs.
The simplest demodulation circuit for amplitude modulation signals
consists of a diode and a capacitor. In a suspended cell, the membrane acts as a capacitor, while there
are two methods in which ion channels can act as diodes to demodulate the
signal.
Ion channels will
normally conduct along a concentration gradient of the particular ion they
transport. If there is a much higher concentration of ions inside the cell than
outside the cell ( or vice versa) making the flow of a particular ion
essentially one direction similar to the flow of electrons through a diode. Additionally, some ion channels move ions
directionally have an intrinsic selectivity filter only allowing excreting or taking up a
particular ion and thus relative to total potential ionic current as a
whole behave as a diode even without a
concentration gradient. Since an ion
needs a characteristic time to be transferred though a particular ionchannel,
the electric field will only have an effect on ion channels that transfer
change in less than half the period of the signal. This has been modelled as an
inductive component in then ion channels response for some time and gives
rise to resonance conditions at well defined frequencies. Although Elnasharty et al only focus on the electric field, I
conclude that the magnetic field will
similarly exhibit resonance effects due to ion cyclotron resonance. These resonance peaks should be detectable in the DEP response of
the cell. If the amplitude of a high-frequency carrier
wave is modulated using a lower
frequency signal, then I would anticipate that where there is a change in
conductance to due to ion channel activity, the net force on the cell would be
due to the superposition of the low-frequency signal acting across the membrane
due to the demodulation effect, plus the effect of the high-frequency
signal. This force will also be felt by
any piezo channels present in the cell.
This high frequency component will
depend solely on the interaction between the medium and cytoplasm, the
membrane having been bypassed at these frequencies. It is therefore possible to
deduct the high-frequency force component by measuring the force acting on
cells when exposed to an unmodulated signal at the carrier frequency. Hence, by
using a low-frequency signal modulated onto a MHz frequency carrier
signal. This is what allowed Elnasharty
et al to observe the DEP spectrum of cells at low frequency and observe
changes to the spectrum when channel blockers and other chemical agents were
used. They observed frequency peaks
both in the low tens of Hz and at about 1Khz.
The precise frequencies depended on the nature of the channel blocker
and/or ionophore.
The work of
both D'InzeoStefano and Elnasharty is useful in that it provides a viable
physical mechanism for interactions of modulated radio frequencies and biology of which there are lots of experimental
observations but few viable explanations.
Whereas essentially their work is interpreted in terms of electric field
gating and component we must never forget that in an EM wave the magnetic
component is inseparable. Both are
candidates for demodulation. We only
have to research the earlier pioneers of radio and the magnetic coherer which
preceded the cat’s whisker to understand this.
Moreover, I will show later there are potentially fewer objections in
terms of signal to noise ratio in the magnetic case.
Further evidence linking the frequencies with ion
channels, initial thoughts.
The average GMF (geomagnetic field) in the USA is some 47.5
micro-tesla. I therefore constructed table 1 below
to show the ion cyclotron
resonance parameters of all the common biological ions, most taken
from Bioengineering and Biophysical
Aspects of Electromagnetic Fields (Handbook of Biological Effects of
Electromagnetic Fields)20 Oct 200 by Ben Greenebaum and Frank S. Barnes (
Chapter 9 Liboff) also found at http://www.sibeonline.com/download/Liboff%20-%20ICR%20interactions%20in%20living%20systems%20-%20SIBE%202013.pdf.
Following this reference, I have obtained the fc/B values for certain
tabulated ions and the rest I have calculated from their charge and molecular
weights. I have then divided
those fc/B values into the given values of tumour treating frequency. When treated in this way each tumour
frequency appears about two orders of
magnitude higher than expected ICR
frequency. One possible explanation for
this is that ICR theory needs to be amended
to take into account dielectric constant
and/or viscosity. I would predict
a quasi- harmonic behaviour for each ion if its angular momentum is to be
conserved as it descends through the selective filter and into the hydrophobic
core. In other words when the ion is
in a totally hydrophobic channel environment it will seem has though it has
lost mass or waters of hydration. I
believe this argument to be justified as follows.
Biological ion
channels are nanoscale transmembrane pores. When water and ions are enclosed
within the narrow confines of a sub-nanometer hydrophobic pore, they exhibit
behaviour not evident from macroscopic descriptions. At this nanoscopic level,
the unfavourable interaction between the lining of a hydrophobic pore and water
may lead to stochastic liquid vapor transitions, see Aryal et al (2014). These transient vapor states are de-wetted ,
i.e. effectively devoid of water molecules within all or part of the pore, thus
leading to an energetic barrier to ion conduction. This process, termed
hydrophobic gating was first observed in molecular dynamics simulations of
model nanopores, where the principles underlying hydrophobic gating (i.e.,
changes in diameter, polarity, or transmembrane voltage) have now been
extensively validated.
Previous
observations of ICR have been in solution.
Calcium ICR can exhibit hyperfine splitting effects due to hydronium and
hydroxyl, see for example but not exclusively Sheykina 2016.
‘Characterisation of weak magnetic field
effects in an aqueous glutamic acid solution by nonlinear dielectric
spectroscopy and voltammetry.’ It is my
contention that when experimenters have attempted to apply ICR frequencies in
biological situations they have used these solutions determined frequencies
which are those of hydrated ions.
Whereas they may be a handful of biological situations where this is
relevant (refs) it is clearly not the
case here. There is another essential
difference also. The Q-factors observed
for ICR elsewhere are low. The bandwidth
is of the order +/- 10% of the
fundmanetal frequncy. In the TTF
case here Q values of apparently between 10^4 and 10^5 are seen at least in
terms of the requirement to produce biofeedback. I will show that the difference can be
understood in terms of the difference between bulk and structured water, ion
cages and dehaydration following for example
Del Giudice. Pazur (2018) also consders calcilum ICR in water cages and
finds the oscillations of the
Belousov–Zhabotinsky chemical reaction are significantly reduced under
Ca²⁺ ICR application. Secondly an “oscillator” of calcium ions appears to
be able to itself couple coherently and predictably to large-scale coherent
regions in water. This system appears able to regulate ion fluxes in response
to very weak environmental electromagnetic fields. See Fulltext
http://www.tandfonline.com/eprint/KYKEqMetHpz7sKwakZct/full.
f (
27.065) Brazil average
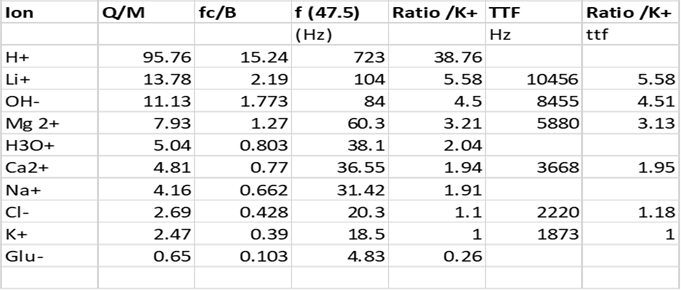

Table 1
It can be clearly
seen that the ratios of frequencies observed from the TTF’S (modulation
frequencies applied to 27 MHz carrier) reference potassium as a base frequency
(Table 1 column 7) are highly compatible
with the ratios obtained from the more classically reported Ion Cyclotron
resonance frequencies (Table 1 column 5). Although it is believed this has
never been attempted by any authors or research groups previously, it is
relatively easy to account for the high harmonic content observed. Several have commented that ICR cannot
properly apply in the hydrated case. There are strong viscous forces on the
ion. Indeed Halgamuge et al (2009) have highlighted the signal to noise ratio
problem with the basic ICR model and has also noted that theoretically true ICR
did not ought to be able to occur for ions in a viscous medium at frequencies
below about 2000 Hz due to the number of collisions per second they are
encountering. Lednev ( ref) amended
the ICR model and came up with the IPR model
which overwhelms the SNR problem
and has similar predicted
frequencies. The same mathematical
prediction can be obtained using a different theoretical approach: the analysis
of the velocity of the damped ion under the influence of the Lorentz force, see
Vincze et al (2008). In both cases,
the prediction of a dependence on specific values for B AC/B DC has been tested
in several experiments.
Liboff and McLeod
(1988) first considered the cyclotron resonance model for channel ion transport
in weak magnetic fields is extended it to include damping losses. Their
model leads to discrete modes of vibration (eigenfrequencies) in the
ion‐lattice interaction, such that ωn = nωc. The presence of
such harmonics is compatible with recent results by Blackman et al. [1985b] and
McLeod et al. [1986] with the interesting exception that even modes do not
appear in their observations. Especially
relevant to the present interpretation in my work, their model has no restriction whatsoever on n.
Further their harmonic formalism is also consistent with another reported
phenomenon, that of quantized multiple conductances in single
patch‐clamped channels.
Liboff et al (2106) have made recent observations of
low-frequency electromagnetic oscillations in water which suggest an inductive
structural component. Accordingly they
assumed a helical basis enabling them to model water as an LC tuned
oscillator. A proposed tetrahedral structure consisting of three water
molecules and one hydronium ion was incorporated into the Boerdijk-Coxeter
tetrahelix to form long water chains that are shown to have resonance
frequencies consistent with observation. Their
model also serves to explain separately reported claims of ion cyclotron
resonance of hydronium ions, in that the tetrahelix provides a built-in path
for helical proton-hopping. For this
reason I shall include hydronium ions in my list of biological ions for later
analysis of Zimmerman and Pasche’s data.
If I take the LC
model, it is logical to suppose the
resonant frequency may depend inversely on the square root of the dielectric
constant. Thus as we descend into
the hydrophobic region epsilon falls from 80 to as low as 2-6. If
ICR or ICR like and water cluster
resonance cohere, as has been suggested by
Del Giudice (ref) we should expect an increase of up to 7 fold in frequency. If the angular momentum of the ion entity
is conserved I would similarly expect a frequency to be proportion to the
inverse of the square root of the effective radius. For a loss of 6 water molecules this
represents approximately a five fold increase in frequency. Much larger water clusters are reported in
biological systems so theoretically this could easily double. For example, often the binding of two protein
molecules seems to be mediated by clustered water. It is known, for example,
that the crystal structure of trypsin and trypsin inhibitor don't fit together
perfectly and the amino acid side chains conflict. In order to form a tight
complex, these side chains must change their conformations. Mobile water
structures along the proteins' surfaces link the two proteins by binding to
each. To do this these water structures
are organized as fragmented dodecahedrons (12-sided figures), 9–15 Ångstroms
long, enough to accommodate 30 or more molecules. There are similar events in
the biochemistry of myoglobin.
Combining the
dielectric constant idea and the conservation
of angular momentum could thus easily account for the observation of an
ICR frequency some seventy times higher than expected. Previously ICR harmonics as high as about 15
have been reported, see for example Pazur (2004) who note ICR for glutamic acid at 4.14 Hz but
note other frequencies worthy of remark are 62, 78 and 94 Hz, being four folds
of the used base ICR resonance frequency 4.14 Hz.
Further, I would perhaps expect there to be
special conditions where higher harmonics still could match the ICR frequency of more than one type of ion simultaneously as in, for example, their lowest common multiple. Since Zimmerman and Pasche’s biofeedback frequency
registration technique relies on stimulating excitable tissue, I would
naturally expect these ‘LCM’ conditions to
produce a strong stimulus. Under
such special conditions one may well have frequencies which drive these ICR’s
in phase with mechanical resonance of other structures within cells or their
organelles.
Furthermore, due to
the vast number of different types and families of ion channels in biology I
would expect an almost pseudo-random distribution of harmonics of each specific
ion’s ICR Frequency depending on type of channel, size and shape of the
selective filter and pitch of the helices involved. For example some channels are more conical
than others. In fact, this is exactly
what the data shows.
It is well known
that the components of ion channels execute coupled movements, see for example
Horn ( 2002). For example; there has to
proper co-ordination between the S4,5 and 6 sub-units in the open and closed
states. There is experimental evidence
to suggest some of these movements are rotational, see Horn (2000).
Thus the channel
itself or its various sub-units will have finite angular momentum and will
hence behave as a harmonic oscillator.
Placing additional
angular momentum on the traversing ions
by means of ICR at its fundamental or harmonic frequencies will lead to superposition behaviour
effectively there will be regions of motional enhancement and regions of
motional restriction depending on the harmonic frequency. Due to the very precise structure and
bonding requirements in a moving ion channel it is plausible to visualise how
high Q responses with in phase and
antiphase dehydrated ion motions might be achieved.
Previous discussions
of the interaction of EMF and biology has only considered ion channel
enhancement (refs). Electromagnetic
fields act via activation of voltage‐gated calcium channels to produce
beneficial or adverse effects, see Pall et al (2013). Usually only calcium channels either inwardly or outwardly rectifying
voltage gated types have been considered (refs). Hence it has been stated and experimentally
shown in some cases that application of ELF ( refs) or even modulated 147 MHz (
ref) causes increased calcium
efflux/influx ( check refs). The ICR
or similar models have been used to explain this on the grounds that ions on
the membrane surface and close to pore entrances are encouraged en-route as it
were. Influx or efflux is encouraged depending on the type of ion channel and the initial membrane or
ligand surface concentration of ions. The frequencies or modulation frequencies employed have exclusively been
low ( Hz or tens of Hz). There is sufficient body of scientific
evidence to suggest that application
of calculated ICR frequencies has real
biological effect, with one of the earliest and most profound papers being that
of Smith and Liboff et al ( 1987)
dealing with Calcium Cyclotron
Resonance and Diatom Mobility. This is
elegant because it shows downstream effects of ICR controlling simple molecular
machinery.
There is also
evidence form the field of plant biology.
Smith et al (1995) tested the ion cyclotron resonance theory of
electromagnetic field interaction with odd and even harmonic tuning for cations
on seeds of Raphanus sativus, var. Cherry Belle. The seeds were exposed to combined parallel
static and sinusoidal 60 Hz, 40 μT peak-peak ac fields turned to the
fundamental, 2nd and 3rd cyclotron resonance harmonics for calcium and potassium
ions. Other seeds were exposed to similar fields tuned to the fundamental and
5th harmonic for magnesium. Concurrent controls consisted of seeds exposed to
the ac field only, and to ambient geomagnetic and stray 60 Hz ac fields. After
21 days plant height, aboveground
weight, root weight, stem diameter, leaf length, leaf width and length/width
aspect ratio were measured and compared to in-group controls. Calcium slowed
germination, potassium speeded it, and magnesium left it unaffected. Calcium
and magnesium tunings were generally stimulatory to growth, while potassium
tuning was inhibitory, except for root weight. Controls (ac only) were
unchanged from the ambient field controls. Fields at the 2nd harmonic were
ineffective, except for potassium 2N, which appeared similar to a weak calcium
effect.
Comisso et al (2005)
studied dynamics of the ion cyclotron
resonance effect on amino acids adsorbed at the interfaces. They reproduced the Zhadin experiment, which consists of the
transient increase of the electrolytic current flow across an aqueous solution
of L‐arginine and L‐glutamic acid induced by a proper low frequency
alternating magnetic field superimposed to a static magnetic field of higher
strength. Further they identified the
mechanisms that were at the origin of the so‐far poor reproducibility of
the above effect: the state of polarization of the electrode turned out to be a
key parameter. The electrochemical investigation of the system shows that the
observed phenomenon involves the transitory activation of the anode due to ion
cyclotron frequency effect, followed again by anode passivation due to the
adsorption of amino acid and its oxidation products.
The relevant
conclusion here was that there will be
the likely occurrence of similar ion
cyclotron resonance (ICR) phenomena at biological membranes and hence the implications not only for common small
ion circulation but also for amino acid circulation in living matter under the consequent impact of environmental magnetic fields.
A useful analogue
for ion channelling and downstream control is to imagine the building a six mile high dam around the
deepest part of the ocean. Now picture what a cell does when it reduces calcium
ions to 20,000-fold lower levels inside the cell than surrounding the cell.
Uncontrolled Ca2+ leaks induce cell death, whereas controlled Ca2+ entry
triggers an enormous array of actions, ranging from secretion to cell division.
Ion channels are the
electrical switches that control these actions. One ion channel directs the
flow of ~10 million ions per second, in turn rapidly changing intracellular
Ca2+ levels. The human genome contains more than 300 genes encoding ion
channels, effectively these are the cell's transistors.
Although demonstrated in plants few are probably
unaware of ICR effects in mammalian and human biology under the
influence of environmental fields other
than what has been reported on Calcium channel effects. Hence probably why Elnasharty et al suggest that the potassium channel too
may be a target for electronic modification as though this in itself were a
rather radical proposition..
However, I will show
herein that not only a numerous different voltage and ligand gated ion
channels for all common biological ions are effected by the Zimmerman and Pasche TTF’s but also
and for the first time, that high harmonics of ICR act in a manner contrary
to that associated with ELF
application. In other words under some
conditions Ca2+ entry can be slowed rather than accentuated. This is entirely consistent with both the
angular momentum hypothesis and the dehydration hypothesis above. Using the same experimental data due to Zimmerman
and Pasche, I will also show that high ICR harmonics also effect amino acid
transporter channels too. Finally, I
will also show that under some conditions K+ can also be suppressed.
Suppressing Ca2+
current will be shown to account for the genetic effects in XCL2 and suppressing of K+ current will
also be shown to be responsible for the genetic effects on PLP2
expression.
There has been
comment by Teplan et al ( 2017) that the
Q factors seen with Pasche and Zimmermann’s TTF’s are unrealistically
high. However if one treats the system
as a mechanically resonant system with
viscoelastic damping and considering the de-wetting phase of ions one can
consider the transition from water to vapour viscosity.
Evaluating the
resonance condition one arrives at
Qv/Qw = Eta w/ Eta v
And it is also known that the viscosity and viscous shear
forces in nanoconfined water can be orders of magnitudes larger than in bulk
water if the confining surfaces are hydrophilic, whereas they greatly decrease
when the surfaces are increasingly hydrophobic. This decrease of viscous forces
is quantitatively explained with a simple model that includes the slip velocity
at the water surface interface, see Ortiz-Young et al (2013).
The two processes
above are sufficient to account for the high Q’S observed.
The ultimate aim is
to know exactly how to control ions in the transport channels of living cells
opens up a fantastic new era and paradigm for both the diagnosis and treatment
of human disease. The advent of the drug
free channel blocker is upon us. Not only that but we may also finally be able
to properly and fully evaluate the true hazard or otherwise of radio
communication systems on biological systems and moreover even design safer such
systems for the future.
Connection with Royal Rife?
There have been so
many versions of the so called Rife machine and so many published frequency
lists that it is virtually impossible to tell which are ‘original’ and which
are ‘fake’.
However one version
of the machine appeared to modulate multiple sidebands at a frequency of about
20-21 KHz onto a 3.3 or 3.8 MHZ carrier
wave. Calculation of this frequency as
an ion yields the 29th
harmonic of proton ICR for a GMF of 47.5
micro-tesla. The machine would not have
had the benefit of modern DDS stability and hence one can postulate that with
frequency drift and jitter it could have occasionally excited ICR in multiple
types of ion channels.
Another version of
the machine was described by one of Rife’s associates, namely John Crane in
1973. He
tried to patent the ‘Frequency Instrument’. Here are some extracts from
the patent application:
"It has been
well known by Rife, myself and others that a specific cancer virus causes the
cancer which was long ago isolated by Royal R. Rife and cancer was cured by
Rife in animals and in clinical tests with people and was published by the
Smithsonian Report for 1944 on pages 193-220 as written by R. E. Seidel, M.D.
(and see U.S. Government Printing Office Publication No. 3781 which has 5
plates of Rife’s microscopes). It was observed that electromagnetic energy
utilizing a frequency of 2127 cycles per second modulated on a carrier wave of
4150 kilocycles (4.15 Mhz) at 200 watts was lethal to cancer.’’
Here the modulation
frequency of 2127 Hz would seem
extremely close to that seemingly causing ICR in chloride channels as used by Zimmerman et al at least from the
simplified interpretation in Table 1 above.
Moreover a quick calculation shows this frequency to be the third
harmonic of the hydrogen ICR, the 20th harmonic of the Lithium ion ICR and the 57th
harmonic of the
calcium ICR assuming an average geomagnetic field of 46.58 micro-tesla, not unreasonable for North
America.
Detailed interpretation of the Zimmerman and Pasche frequencies.
Because the
geomagnetic field in Sao Paulo is
estimated to be between 20-30 micro-Tesla
it is not appropriate to develop a detailed interpretation either from
Table 1 above or by normalisation because the field will vary on a day by day
basis. I thus considered in much
greater detail the downloadable files,
there are three of which, described as;
Breast tumour treating frequencies, http://drchrisbarnes.co.uk/BREAST.pdf and HCC (liver) treating tumour frequencies. http://drchrisbarnes.co.uk/HCC.pdf and Random frequencies which did
not provoke effect, http://drchrisbarnes.co.uk/RAND.pdf. These files are used as follows;
The extreme left
column is the so called TTF ( tumour treating frequency as identified by the reflex response of the patient directly or by
physiological monitoring of changes to
patient vital signs such as heart rate and blood pressure. The remaining columns are the precise ICR frequencies and
harmonics for the known common biological
ions and common amino acids at the GMF according to hydrogen. Where there are precise or extremely close
numeric (frequency) matches between the TTF and the ICR harmonic this has been
indicated/highlighted in green. Lesser matches in yellow and lesser matches
still in orange. No match is just left
as bare print, black on white.
Plugging in a value
of 25uT for the GMF yields approximately
380 Hz for the first ICR value of the proton.
In the ‘breast’ file the nearest frequency to this is 414.817 Hz. In the HCC file the nearest frequency 410.231
Hz. I thus interpret these frequencies
as being the fundamental ICR for protons and I interpret the differences not as
‘patient specific’ as suggested by Zimmerman and Pasche et al but rather simply
being due to a difference in the local
GMF when the treatment was given.
Accordingly dividing these two frequencies by Fc/B ( 15.24)
for the proton yields the precise value of GMF in each case, namely 27.21895858 uT in the Breast file and 26.91804 uT in the case of the HCC file. I the utilise
these GMF’s to calculate the expected fundamental ICR frequencies for a
significant number of other common
relevant biological ions including: H+, Li+, OH- , H3O+,
Mg2+, Ca2+, Na+, Zn2+, Cl-, K+ and a number of small amino acids. I then divide these fundamental frequencies
into each of the stated 194 TTF frequencies to find the harmonic number for
each ion. I define a harmonic as being
valid if it falls as an integer or close thereto. For higher harmonics I
allowed a maximum deviation of +/- 0.1 on the harmonic number. I then count the number of harmonics for each
specific ion which fulfils this chosen condition. I have set no upper bound on harmonic
number but essentially my chosen precision gets sharper and sharper in an
arithmetic progression. The results
are available in XL spreadsheets.
Proton ICR harmonics from 1-45 are observed. Much higher harmonics of heavier ions and
amino acids are observed, tantamount with the dehydration hypothesis
above.
The random file
contains 237 random frequencies and it is stated none are closer than .5 Hz of
an actual treatment frequency. For the
random file I took the GMF as being the average of the GMF for the two
treatment files. I used the same
criteria for the definition of an ion specific ICR harmonic. The result is available in an XL
spreadsheet.
For all three files
I then went on to calculate the percentage of frequencies that fulfilled ICR or
ICR harmonic conditions for each specific ion and have showed these results in
a separate spreadsheet, an extract of which is included in table 2 below:
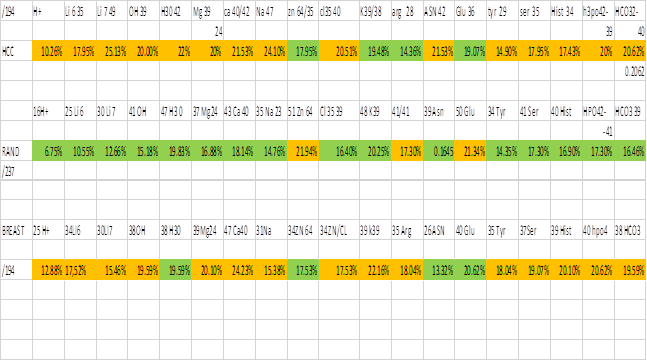
I have used green to
represent a low or lowest percentage for the tabulated ion and orange to
represent high and highest percentages.
It can be clearly seen that the ions which give rise to action
potentials in excitable tissue have more ICR harmonics in the treatment files
than in the random file. This is totally
consistent with perturbation of ions, their channels, the ICR resonance
condition and /or a downstream event being the cause of the biofeedback
registration in the treatment cases and the lack of registration upon
application of random frequencies.
There are also some far more profound observations which I will discuss
later in this present paper. I also
considered hydrogen polarisation models, see
and was unable to fit the results in any way, see Halgamuge et al (2009) .
Can ion channel modulation explain Zimmerman’s results?
Zimmerman et al
categorically state that there are known known mechanisms for their results
which sadly downplays their excellent work and makes their system less likely
to be commercially exploitable.
Human nature tells
us if you buy something you want to know how it works. Like a drug you don’t
know how or why it works then it could simply be placebo! I have included Zimmerman’s graphic to show
where they are at.
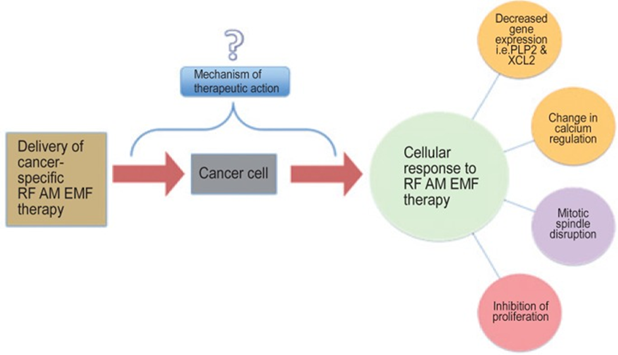
I will spend the
rest of this paper attempting to explain Zimmerman et al’s observations of
downregulated PLP2 and XCL2 genes and mitotic spindle disruption especially
with regard to HCC liver cancer.
XCL2 and Calcium
regulation
XCL2 is the other member of the C-chemokine
subfamily, XCL1 being the first and more well-known member. Further,
XCL2 is responsible for G protein-coupled receptor activation while the
dimeric form is important for GAG binding. Despite their high structural
similarity, XCL2 displays a slightly higher affinity for heparin than XCL1.
Because their in vitro functional profiles are virtually identical, distinct
physiological roles for XCL1 and XCL2 are probably encoded at the level of
expression, see Fox et al (2014). Since Chemokines are immune modulators it is
possible the expression of this gene fell in response to there being less
overall metastases. For instance,
the Chemokine Network has a role in the
Development and Progression of Ovarian Cancer
and is hence a potential Pharmacological Target, see Barbieri et al
(2009). Chronic inflammation is a also a
risk factor for several gastrointestinal malignancies, including colorectal
cancer. Recent epidemiological studies and clinical trials demonstrate that
long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) markedly
reduced the relative risk of colorectal cancer. Chronic inflammation associated
with development of cancer is partly driven by the chemokine system. They are
also involved in gliomas, melanomas,
and breast cancer, see Memtlein et
al ( 2013) who also discusses Chemokines
as a possible target.
XCL2 and CX3CL1
expression in lung cancers and adjacent non-cancerous tissues has been
studied by Zhou et al (2016) using quantitative PCR and ELISA. The
relative expression of both chemokines in lung cancers in different
pathological stages was compared by immunohistochemical assay. The expression of XCL2 and CX3CL1 increases
with increasing degree of malignancy, indicating that both chemokines might be
important targets in gene therapy for lung cancer. Their study demonstrated that XCL2 and
CX3CL1 expression in lung cancers were significantly higher compared to adjacent
normal tissues. Moreover, expression of both chemokines was significantly
stronger with higher pathological stages.
They speculated that XCL2
and CX3CL1 synergistically promote the development of lung cancer.
Tthe paradigm of
cancer development and metastasis has been redefined to encompass a more
comprehensive interaction between the tumor and microenvironment within which
the tumor cells reside. Despite the realization that this more comprehensive
relationship has changed the current paradigm of cancer research, the struggle
continues to more completely understand the pathogenesis of the disease and the
ability to appropriately identify and design novel targets for therapy.
Chemokines and chemokine receptors in general
are being investigated for their role in tumor development and
metastasis and may prove to be useful therapeutic targets. The chemokine family
is a complex network of molecules that are ubiquitously expressed and perform a
variety of functions most notably regulating the immune system. Here we review
the importance of chemokines in the tumor-stromal interaction and discuss
current concepts for targeting the chemokine network.
XCL2 encodes for a
protein that enhances chemotactic activity for lymphocytes and downregulation
of XCL2 has been shown to be associated with good prognosis in patients with
breast cancer (Teschendorff et al, 2007; Teschendorff and Caldas, 2008).
XCL2 is structurally similar to XCL1,
displaying metamorphic interconversion. The monomeric form of XCL2 activates
XCR1 and has a similar potency to XCL1.
Perhaps the most important question here is
can any direct link be established between RF ion channel
modulation and XCL2 expression or is the fall in the latter simply a consequence
of cancer cell destruction by another mode.
The papers in the Barbault and Zimmerman family categorically state that
effects could not be elicited at other modulation frequencies so we are left to
consider whatever mechanism must differ from that of Kirson et al.
The drugs Etanercept
and Infliximab are capable of
Modulating Proinflammatory Genes in Activated Human Leukocytes. Etanercept is a recombinant human tumor
necrosis factor
receptor [p75]: Fc
fusion protein. Infliximab is a chimeric anti-tumour necrosis factor α
monoclonal antibody. Two chemokines
are downregulated by etanercept, XCL1 and XCL2, have been identified as two
isoforms of lymphotactin.
Infliximab but not
etanercept induces apoptosis in lamina propria T-lymphocytes from
patients with Crohn’s disease an autoimmune condition. Conversely, anti-tumor Necrosis Factor
Alpha (Infliximab) attenuates Apoptosis, Oxidative Stress, and Calcium
Ion Entry Through Modulation of Cation Channels in Neutrophils of Patients with
Ankylosing Spondylitis, an auto inflammatory disease, see Ugan et al (2016).
In conclusion, their
current study suggests that infliximab is useful against apoptotic cell death
and oxidative stress in neutrophils of patients with AS, which seem to be
dependent on increased levels of intracellular Ca2+ through activation of TRPM2
and VGCC.
TRPM2-mediated Ca2+
influx induces chemokine production in monocytes that aggravates inflammatory
neutrophil infiltration.
Transient receptor
potential (TRP) channels, have been implicated in tumour cell migration and the
metastatic cell phenotype, see Prevarskaya (2011).
TRPV1 and TRPV4
channels provide Ca2+ entry pathways in HepG2 liver cancer cells. HGF/SF increases Ca2+ entry via TRPV1,
but not via TRPV4. This rise in [Ca2+]i may constitute an early response of a
signalling cascade that gives rise to cell locomotion and the migratory
phenotype, see Vriens et al (2003).
Store‐operated
calcium entry (SOCE) is the main Ca2+ influx pathway involved in controlling
proliferation of the human hepatoma cell lines Huh‐7 and HepG2, see Boustany et al (2008).
Now we have a
putative link between inflammation, invasion and metastasis and intra-cellular
Ca2+ ion concentration. It seems
the TTF modulated signal is acting
rather like Infliximab and in this
case causing Ca2+ efflux or slowing
Ca2+ entry, hence downregulating XCL2.
Either way the action, brought about by the tumour treating frequencies
modulated onto the 27.12 MHz signal is
deep within the hydrophobic part of the channel as identified by the high ICR
harmonics present. The higher ICR Harmonics for calcium seem to be evenly
spread across the TTF frequency spectrum.
This is consistent with the progressive dehydration of calcium as it passes through the pore and as
documented in the literature and is equally consistent with both the dielectric
and angular momentum models. Mitosis
or S-phase in cancer cells is usually
associated with Ca2+ entry which augments depolarisation and provides an
internal store of calcium to operate calcium operated potassium efflux channels
leading to cell shrinkage before division.
Hence interfere with Ca2+ entry will interfere with mitosis which is
what is observed. Running in tandem the
TTF’s also block sodium entry see Table 2, augmenting the same mechanism.
PLP2
This gene also
called Phosducin-like Protein 2 encodes
an integral membrane protein that localizes to the endoplasmic reticulum in
colonic epithelial cells. The encoded protein can multimerize and may function as
an ion channel. A polymorphism in the promoter of this gene may be linked to an
increased risk of X-linked mental retardation. A pseudogene of this gene is
found on chromosome 5. [provided by RefSeq, Jan 2010]
PLP2 is usually
expressed in yeasts and hepatitis and several other viruses. A
functional role for PLP2/A4 has
been suggested by Lee et al (2004) in the chemotactic processes via CCR1.
Proteolipid protein
2 (PLP2) has been shown to be upregulated in several cancers, including breast
cancer, hepatocellular carcinoma, osteosarcoma, and melanoma. PLP2 specifically
binds to phosphatidylinositol 3 kinase to activate the protein kinase B pathway
to enhance cell proliferation, adhesion, and invasion in melanoma cells , see
Ding et al (2015). They speculated that PLP2 exhibits oncogenic potential.
However, they also reported that the regulatory mechanisms of PLP2 in cancer
cells remain unclear.
Sonoda et al (2010)
were able to show that knockdown of PLP2 with an artificial microRNA reduced
growth and metastasis in B16BL6 melanoma cells.
Cadmium a heavy
metal poison is now shown as being able to regulate gene expression. It induces modifications of the expression
level of genes coding for members of stress response-, mitochondrial
respiration-, MAP kinase-, NF–κB-, and apoptosis-related pathways. Longo
et al (2012) showed that PLP2
(proteolipid protein-2)was a novel member of the list of Cd-upregulated
genes. Further, through the application of transfection
techniques with specific antisense oligonucleotides, they demonstrated that such over-expression may be
an upstream event to some of the changes of gene expression levels already
observed in Cd-treated cells, thus unveiling new possible molecular
relationship between PLP2 and genes linked to the stress and apoptotic
responses.
Del Marmol ( PhD
Thesis 2016) has shown that Plp2
amplifies the magnitude and slows down the kinetics of the Piezo1
mechano-sensing ion channel. Another protein, Cd63, is also a transmembrane
protein that only amplifies the magnitude of Piezo1 currents, with no
modification of its kinetics, in heterologous expression. Given the remarkably
large set of functions that have been attributed to Piezo channels 6,7,8,9 in
the very few years since its discovery, and how little we still know of its
functional mechanisms, the identification of novel modulators provides a
crucial next step in elucidating the molecular basis of mechano-sensation.
One recently
published study proposed some intriguing effects of music on the well-known
breast cancer cell line MCF-7. The authors of this study reported that exposing
MCF-7 cells to sound pressure of 70–100 dB induces changes in cell cycle, cell
viability and morphological changes. Despite this interesting observation,
perhaps a better understanding of Piezo
1 and Plp2 will lead to an explanation
for these findings in vitro?
Mechanical tension
generated within the cytoskeleton of living cells is emerging as a critical
regulator of biological function in diverse situations ranging from the control
of chromosome movement to the morphogenesis of the vertebrate brain, see
Chicurel (1998).
Mutant forms plp2-ts cells have increased sensitivity to
cytoskeletal destabilizing drugs such as
Benomyl and Latrunculin, see
Stirling et al (2007), and are larger than wild-type cells. (A) Benomyl
and latrunculin sensitivity of plp2-1 and plp2-2 mutants relative to wild-type
(PLP2) cells, as determined by relative clearance caused by drug-inoculated
paper discs when grown at the semi permissive temperature of 30°C.
Latrunculin A is
isolated from the nudibranch Chromodoris sp. Houssen et al (2006) studied its
effects on the electrophysiological
properties of cultured dorsal root ganglion neurones. Latrunculin A alone had no effect on
intracellular Ca2+. However, under
voltage-clamp conditions, significant and dose-dependent suppression of K+
current was seen with 10–100 μM latrunculin A. See, Na+/Ca2+ selectivity in the bacterial
voltage-gated sodium channel NavAb Research article Biophysics Computational
Biology Ben Corry Published February 12, 2013.
I thus hypothesise
that the frequencies employed have a similar effect on PLP2 to Latrunculin and
this is because they effectively suppress K+ current. Hence we have the link for reduced PLP2 and
another explanation of the observed damage to the mitotic spindle. With TTF’s the K+ efflux may be being
suppressed as a product of the reduced calcium influx but also directly by
blocking of the Potassium channel in the selective filter and/or hydrophobic
regions of the channels. Once again,
this is entirely consistent with the theory.
Besides
electromagnetic waves it may even be possible to produce similar effects with
sound or modulated ultrasound.
The biological
effects of electromagnetic waves are widely studied, especially due to their
harmful effects, such as radiation-induced cancer and to their application in
diagnosis and therapy. However, the biological effects of sound, another physical
agent to which we are frequently exposed have been considerably disregarded by
the scientific community. Although a number of studies suggest that emotions
evoked by music may be useful in medical care, alleviating stress and
nociception in patients undergoing surgical procedures as well as in cancer and
burned patients, little is known about the mechanisms by which these effects
occur. It is generally accepted that the mechanosensory hair cells in the ear
transduce the sound-induced mechanical vibrations into neural impulses, which
are interpreted by the brain and evoke the emotional effects. In the last
decade; however, several studies suggest that the response to music is even
more complex. Moreover, recent evidence comes out that cell types other than
auditory hair cells could response to audible sound. However, what is actually
sensed by the hair cells, and possible by other cells in our organism, are
physical differences in fluid pressure induced by the sound waves. Therefore,
there is no reasonable impediment for any cell type of our body to respond to a
pure sound or to music. Hence, the aim of the present study was to evaluate the
response of a human breast cancer cell line, MCF7, to music. The results'
obtained suggest that music can alter cellular morpho-functional parameters,
such as cell size and granularity in cultured cells. Moreover, our results
suggest for the 1sttime that music can directly interfere with
hormone binding to their targets, suggesting that music or audible sounds could
modulate physiological and pathophysiological processes. I hypothesise that
Piezo 1 and 2 are responsible for the above.
Further Discussion and Advanced Analysis.
It is documented
that potassium loses all water in the selectivity filter.
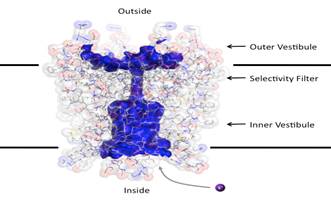
This is entirely
consistent with the great majority of close integer ICR harmonics for potassium falling at the
highest TTF frequencies. I propose
antiphase interference in the selectivity filter limits the K+ current
suppressing PLP2 causing loss of cytoskeleton and interference with mitosis. I further propose that disturbance to potassium
current suppresses kinesin motors. This is documented at least in the neuronal
case, see Barry et al (2013).

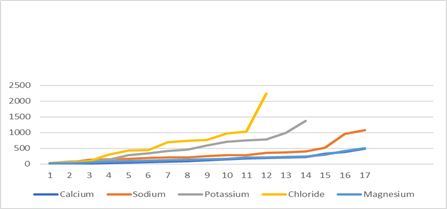
Plot 1
On the other hand the literature teaches us that sodium ions are
mostly hydrated in the wide pore and for the most part one can see from Plot 1 above that for the greater part of the
TTF frequency range the sodium curve falls well below potassium indicating more
hydration.
Additionally, the
literature teaches that chloride in ion channels is totally dehydrated and for 78% of the TTF
range we see that chloride has higher integer like ICR harmonics than all the
other ions exactly as predicted by both the dielectric model and/or the angular momentum model.
Finally, the
literature teaches us to expect that Calcium should be slightly less hydrated than magnesium upon deeper
progression into the pore with 3-4 water molecules as opposed to 6 and once
again this appears to borne out by the result wherein from some 60% through the
TTF frequency range from low to high,
calcium overtakes magnesium in terms of the magnitude of near integer ICR
harmonic values. Although at very high
TTF frequencies magnesium appears as though it is overtaking calcium as it
starts to be stripped of water in the selectivity filter.
Whereas the
fundamental ICR frequency will be a property of ions in isolation, the harmonic
frequencies will be very much a product of ion and channel in tandem. There are multiple types of each channel expressed due to genetic variation
in excitable tissue and tumour tissue.
Plot 1 is an a sense
a snapshot of total ‘channelopathy’ for HCC.
We should therefore expect to see some similarities form what is known
of ion channels in general and some
differences in the case of breast cancer.
Additionally, different genetic variations of ion channels are expressed
and utilised by cancer cells at
different stages of metastasis.
 Plot 2
Plot 2
Considering plot 2
then, the result for breast cancer. The
same general trends are present Chloride, Potassium and Sodium as with HCC Plot
1. At
first sight, however, on the scale of plot 2 calcium and magnesium
appear indistinguishable. It is thus
instructive to plot them on a more appropriate scale. See Plot 3 below:
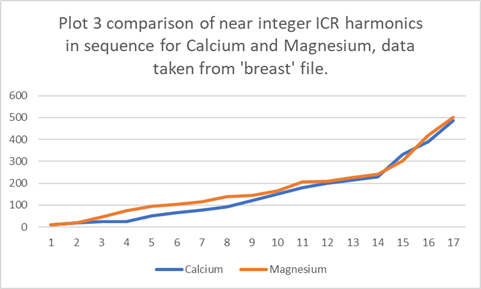
The literature
teaches us that magnesium has 6 water molecules in the pore whereas calcium has
7. I would this expect calcium to show
lower near integer ICR harmonics then magnesium which is exactly as is
observed. Moreover, magnesium
loses more water in the selectivity
filter, borne out by its slight tendency to overtake calcium at the very
highest TTF’s.
From between circa
30-50% the TTF frequency range magnesium
is higher than calcium by a factor of circa 1.56. The radius of the entity has increased by a
factor of about 1.16. Thus the volume of
the entity has increased by 1,16^3 =1.56.
A near perfect agreement.
Furthermore if I
treat the system as a spherical mass and spherical capacitor this is totally supportive of the combined dielectric
and angular momentum hypothesis which I initially advanced.
Voltage gated proton
channels are almost unique in that the proton is transported as simply the
native bear entity and not as H3O+ as in
solution (ref). This is the case even in
water filled gramicidin pores ( ref). I
would thus expect the sequential plot
for near perfect ICR harmonic fits to be virtually linear across the range of
TTF’S.
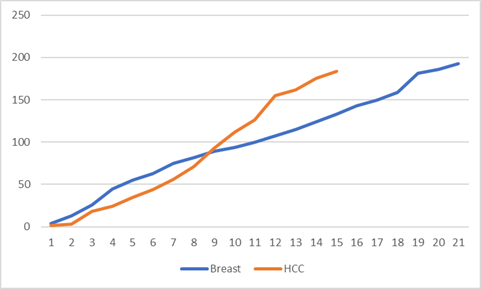
Plot 4 : Near perfect ICR harmonic fits TTF range
frequency number versus sequence number
It is seen from plot
4 that there is considerably more linearity when compared with ions that suffer
staged dehydration through the channel, i.e plots 1-3. This is exactly as predicted.
Using the specific frequency files to inform on specific cancers
By extension of the
hypotheses developed and tested above one can make the following additional and
testable assumption which is that ICR at the fundamental frequency only
requires an ion in water as for example with observations on glutamic acid
(refs), but that very large ICR harmonics are only observed with ion channels
where the water structure is very different from the bulk and/or where there is
angular momentum coupling. There is
some limited evidence in the literature of ICR at low to moderate harmonic
frequencies without reference to ion channels and I suspect this is because
these are the ICR frequencies associated
with smaller than usual water clusters.
Extending the above
assumption to its natural conclusion suggests that the more close to integer
higher harmonic frequencies of ICR there
are present for a particular ion channel in a particular patient with a particular
cancer then the more of these channels there will be physically expressed. This is highly testable by referring to
table 2.
Pasche and Zimmerman
have stated that the random frequencies did not elicit a biofeedback response
in any patients and moreover that the TTF frequencies did not elicit a blood
pressure or pulse rate biofeedback response in patients without cancer.
If we consider the
random frequencies then all we can do is
to calculate the harmonics of ICR and
see where they fall. It is logical
to suppose that there will be genetic variations of ion channels expressed in the excitable
tissue of cancer patients which are also expressed in their tumours. Therefore the immediate upshot of this would
be to expect to find a greater percentage
of very close or reasonably close integer fits to ICR harmonics related to excitable tissue
types in the HCC and Breast cancer treatment files than in the random file. The
voltage ion channels associated with muscle include for instance; sodium,
calcium, magnesium, chloride and proton.
All of which show up significantly
more very close or reasonably
close integer fits to ICR harmonics in
both the HCC file and the breast file than in the HCC file, see figure 2. Very interestingly zinc channels appear to
be depleted in both the cancer TTF files in contrast with zinc channels
expected on a random basis, circa 17.5% fits are found as opposed to circa
22% in the random case. Costello and Franklin (2014) have
discussed the basis is presented for
characterizing HCC malignancy as zinc transporter ZIP14-deficient tumours, and
its requirement to prevent zinc cytotoxic effects on the malignant cells. They
also discuss the potential for an efficacious zinc treatment approach for HCC. In this respect not only are TTF’s shown to
be a treatment modus operandi but also one of diagnosis. Indeed,
zinc deficiency may be a hallmark of several cancers.
Besides cell
membrane channels we may also consider TTF activation of mitochondrial
channels. The main channel of interest
is VDAC1 which favours chloride in the open state and small cations in the closed state.
Inhibiting VDAC1 cause apoptosis. The
proapoptotic effect of VDAC1 is due to its physical interaction with the
IP3 receptor and to its formation of the molecular route for transferring Ca2+
signals to mitochondria in apoptosis. I
postulate that high ICR harmonic chloride TTF’s favour the closed (inhibited)
state. It is very exciting to note that
the anti-fungal drug Itraconazole inhibits in the same manner and has recently
been shown highly effective against bowel cancer. I view TTF as the physical equivalent of this
drug in the case of this ion channel.
I will now deal
specifically with HCC and breast cancer in turn.
HCC Specific Observations
Referring then to
table 2, Sodium channels would appear to be far more expressed in the HCC case
than the breast case, 24.1% as opposed
to 15.38% of the total 194 treatment frequencies considered give very close or reasonably close integer fits to ICR harmonics. ASIC1α is overexpressed in HCC
tissues and associated with advanced clinical stage. is overexpressed in HCC
tissues and associated with advanced clinical stage. ASICS can non-selectively transport sodium. Scn2a1 sodium channel is also involved in
HCC, see Zuniga-Garcia (2015). NaV sodium channels are known to be
associated with proliferation, see Rao
et al (2015). However, no specific
reference can be found to sodium channels of any specific kind being solely
associated with HCC.
Again, referring to
Table 2 there are an apparent large expression
(25% of the TTF’s) of Lithium channels, some 10% greater than random. Whether there is a specific human lithium
channel is unclear ( check) but ASIC1 is known to be an excellent lithium
transporter and is know to be overexpressed in HCC with poor outcome, see Jin et al (2015) and Jin et al (2017). Strangely lithium has been used in the
treatment of some human cancers but is known to cause changes in Micro RNA so
could be a double-edged sword?
Both magnesium and
chloride show approximately 20% of the total 194 frequencies as close or
reasonably close integer harmonic fits
and calcium 21.5%, some 3.4% greater than random. All three of these well exceed the results
for the random file. HCC CSCs
overexpress the calcium channel α2δ1 subunit, see Sainz (2013). Calcium channel blockers have been used in
HCC treatment ( refs). Cell migration
and invasion are two prerequisites for tumor metastasis, in which TRPM7 in HCC
plays an important role, see Chen et al
(2016). Chloride intracellular channel
1 participates in migration and invasion of hepatocellular carcinoma by
targeting maspin, see Wei et al (2014).
Relative to other
neutral amino acid transporters, the expression levels of ASCT2 and LAT1, are
co-ordinately elevated in a wide spectrum of primary human cancers. There seems to be a greater percentage of
asparagine harmonics in the HCC case and the ASCT2 transporter would be the
candidate here. Likewise, they can
transport serine also found to be elevated, see Table 2.
Next, I consider
small amino acid ions. Serine and glutamine and arginine appear to
be suppressed relative to the random expectation. Asparagine appears to be elevated.
Hirayama et al
(1987) studied plasma amino acid levels in 23 patients with HCC in comparison with 16 normal subjects and 17
patients with liver cirrhosis. Patients with hepatocellular carcinoma had
elevated levels of the aromatic amino acids and lowered levels of the
branched-chain amino acids, as seen in liver cirrhosis; however, they had
lowered levels of alanine and glutamine as compared with normal subjects and
with liver cirrhosis patients. Following treatment with intraarterial
chemotherapy and/or transcatheter arterial embolization, plasma levels of
alanine and glutamine recovered. These results suggest that the consumption of
alanine and glutamine increase in hepatocellular carcinoma. Moreover, among the other amino acids,
asparagine, citrulline, ornithine, and cysteine were also elevated.
Thus, I have shown
the TTF technique not only to be a treatment technique but also a powerful
diagnostic technique with the power to make a certain amount of differential
diagnosis.
Breast Cancer Specific Observations
In the case of the
breast cancer file and near of very near harmonic ICR fits, I conclude that the
three most overexpressed ion channels are Calcium, 24.2% of TTF, Potassium
22.1% of TTF, and Magnesium 20.1% of TTF.
These represent increases of 6%,5,8% and 3.22% over the randomly hit ICR
harmonics.
Interestingly,
Tamoxifen (Tx) has been used in breast cancer treatment and prophylaxis because
of its antiestrogenic activity however
it has also been shown to inhibit
voltage-gated calcium current and contractility in vascular smooth muscle from
rats, see Song et al (1996).
Store-operated
calcium (Ca2+) entry (SOCE) mediated by STIM/Orai proteins is a ubiquitous
pathway that controls many important cell functions including proliferation and
migration. STIM proteins are Ca2+ sensors in the endoplasmic reticulum and Orai
proteins are channels expressed at the plasma membrane. The fall in endoplasmic
reticulum Ca2+ causes translocation of STIM1 to subplasmalemmal puncta where
they activate Orai1 channels that mediate the highly Ca2+-selective Ca2+
release-activated Ca2+ current (ICRAC). Whereas Orai1 has been clearly shown to
encode SOCE channels in many cell types, the role of Orai2 and Orai3 in native
SOCE pathways remains elusive. Motiani et al (2010) analyzed SOCE in ten breast cell lines picked
in an unbiased way. They used a combination of Ca2+ imaging,
pharmacology, patch clamp electrophysiology, and molecular knockdown to show
that native SOCE and ICRAC in estrogen receptor-positive (ER+) breast cancer
cell lines are mediated by STIM1/2 and Orai3 while estrogen receptor-negative
(ER−) breast cancer cells use the canonical STIM1/Orai1 pathway. The ER+
breast cancer cells represent the first example where the native SOCE pathway
and ICRAC are mediated by Orai3. Future studies implicating Orai3 in ER+ breast
cancer progression might establish Orai3 as a selective target in therapy of
ER+ breast tumors.
I have already shown
that the TFF’s are acting in a way either to block calcium influx or cause
efflux, see earlier.
Abdul et al (2003)
working with MCF 7 breast cancer cells showed that some 85% had high or
moderate expression of Kv 1.3.
Potassium channel openers enhanced their proliferation whereas potassium
channel blockers dequalinium and amiodarone showed a remarkable 90%
inhibition. I have already suggested that the TTF
frequencies employed have a similar effect on PLP2 to Latrunculin and this is because they
effective suppress K+ current. Hence not
only did I provide the link for reduced PLP2 and offer another
explanation of the observed damage to
the mitotic spindle but this type of
argument is now also supported from the above.
Moreover Woodfork et al (1995) investigated nine
different potassium channel antogonists
with regard to differential
sensitivity of cell proliferation and cell cycle progression and conclude
that ATP‐sensitive potassium
channels in these human mammary carcinoma cells reversibly arrests the cells in
the GO/G1 phase of the cell cycle, resulting in an inhibition of cell
proliferation.
Trapani et al (2013)
discuss magnesium. In vivo, magnesium deficiency, and the resulting
inflammation, can trigger both anti- and pro-tumour effects. Recent
experimental evidence indicates that altered expression of the transient
receptor potential melastatin, type 7 (TRPM7) epithelial magnesium channel is a
frequent finding in cancer cells and human tumour tissues, and correlates with
cell proliferation and/or migration.
Guilbert et al (2009) also provide
evidence that TRPM7 is required for breast cancer cell
proliferation. This supports the notion
of it showing up in the TTF spectrum.
A few references
discuss raised sodium channel expression in breast cancer but this does not appear
to be borne out for the TTF file provided.
Finally, I
considered some common amino acid ions as there transporters are also common
and important in biology. Regarding
the Breast TTF file I show that Tyrosine and Histidine are some 4% higher than
the random expectation, serine is come 2% higher and glutamine and
asparagine.
The observation
regarding serine is interesting.
Serine is of course a component of the protein kinase C (PKC) family of
serine/threonine kinases has been intensively studied in cancer since their
discovery as major receptors for the tumor‐promoting phorbol esters
(ref). PKC comprises 10
phospholipid‐dependent serine‐threonine kinases grouped into three
subclasses: the “classical” (PKC α, βI, βII, and γ), which
can be stimulated by Ca2+ and diacylglycerol (DAG) or phorbol esters; the
“novel” (PKC δ, ε, η, and θ), which can be activated by
diacylglycerol or phorbol esters but are Ca2+ independent; the “atypical” (PKC
ζ and λ/ι), which are unresponsive to Ca2+, diacylglycerol, and
phorbol esters. The structure of classical PKCs includes four conserved domains
(referred as C1–C4) interrupted by five variable regions (V1–V5). The C1 region
contains cysteine‐rich zinc‐finger‐like motifs responsible
for phosphatidylserine, DAG, and phorbol esters binding. An autoinhibitory
pseudosubstrate (Ps) sequence is located at the N‐terminal region of PKCs
that is involved in autoinhibition. The C2 region in classical PKCs is rich in
acidic residues and binds Ca2+. The C3 and C4 regions form the ATP‐ and
substrate‐binding lobes. Novel PKCs have an altered C2 region unable to
bind Ca2+, and atypical PKCs are insensitive to Ca2+ and have only one
cysteine‐rich zinc‐finger‐like motif that is unable to bind
DAG or phorbol ester.
Next considering
tyrosine, mapping of homozygous deletions on human chromosome 10q23 has led to
the isolation of a candidate tumor suppressor gene, PTEN, that appears to be
mutated at considerable frequency in human cancers. In preliminary screens,
mutations of PTEN were detected in 31% (13/42) of glioblastoma cell lines and
xenografts, 100% (4/4) of prostate cancer cell lines, 6% (4/65) of breast
cancer cell lines and xenografts, and 17% (3/18) of primary glioblastomas. The
predicted PTEN product has a protein tyrosine phosphatase domain and extensive
homology to tensin, a protein that interacts with actin filaments at focal
adhesions. These homologies suggest that PTEN may suppress tumor cell growth by
antagonizing protein tyrosine kinases and may regulate tumor cell invasion and
metastasis through interactions at focal adhesions. The
HER-2 tyrosine kinase pathway targets estrogen receptor and promotes
hormone-independent growth in human breast cancer cells, see Pietras et al
(1995). Of course tyrosine is one of
its major components and so we have
another link here.
Finally, considering
glutamine which appears to be in short supply rather as zinc. These findings suggest that dietary GLN
supplementation suppresses mammary carcinogenesis by activation of apoptosis in
tumor cells and this probably is a result of GSH down-regulation. Todorova et
al (2004) have shown that at least in
rats, dietary GLN supplementation
suppresses mammary carcinogenesis by activation of apoptosis in tumour cells
and this probably is a result of GSH down-regulation.
Once again, I have
highlighted the depth and power of the TTF technique.
Link between mechanical models, solitons and ICR
frequencies.
The first evidence I
shall borrow is from a discussion of action potential in neuronal membrane, see
El Hady and Machta (2015). Many diverse
studies have shown that a mechanical displacement of the axonal membrane
accompanies the electrical pulse defining the action potential (AP). In their model, mechanical displacements
arise from the driving of surface wave modes in which potential energy is
stored in elastic properties of the neuronal membrane and cytoskeleton while
kinetic energy is carried by the axoplasmic fluid. Further surface waves are driven by the
travelling wave of electrical depolarization characterizing the AP, altering
compressive electrostatic forces across the membrane. This driving leads to
co-propagating mechanical displacements, which we term Action Waves (AWs). The shape of the AW that accompanies any
travelling wave of voltage, is in
excellent agreement with results from several experimental systems. This model is useful in understanding the
effects of TTF as there would seem to be very predictable phase relationships. This can link ICR frequencies and MT
mechanical resonance ideas.
JA Tuszyński
(2004) has also discussed ionic waves in actin filaments. Gelens et al (2014) discuss the link between ionic waves and
mitotic waves in theor paper ‘Spatial
trigger waves: positive feedback gets you a long way.’
Prindle et al (2015)
show that ion channels conduct long-range electrical signals
within bacterial biofilm communities through spatially propagating waves of
potassium. These waves result from a positive feedback loop, in which a
metabolic trigger induces release of intracellular potassium, which in turn
depolarizes neighbouring cells. Propagating through the biofilm, this wave of
depolarization coordinates metabolic states among cells in the interior and periphery
of the biofilm. Deletion of the potassium channel abolishes this response. As
predicted by a mathematical model, we further show that spatial propagation can
be hindered by specific genetic perturbations to potassium channel gating.
Together, these results demonstrate a function for ion channels in bacterial
biofilms, and provide a prokaryotic paradigm for active, long-range electrical
signalling in cellular communities.
Becchetti (2011)
provides a convincing demonstration that ion channels modulate cell
proliferation which ultimately relies on results showing that their activity is
absolutely key to regulation of the cell
cycle checkpoints.
Little wonder then
that TTF’s via their here proven interference on ion channels alter the mitotic
spindle. Some have attempted to show
that the TTF mode of interaction is
purely one of mechanical resonance. The
fact that biology exhibits long range solitonic transport and positive
feedback means we cannot escape the TTF
interaction with the ion channel, whether chicken or egg.
Conclusions
- A new hypothesis of ICR allowing for
very high harmonic frequencies to develop
upon dehydration due to lowered dielectric constant and
conservation of angular momentum is developed and tested. The importance of GMF ( local
geomagnetic field) is emphasised.
- A plausible hypothesis for the
involvement of ion channels in the TTF technique of Zimmerman and Pasche
had been tested and strongly
supported.
- The system has been
shown to be different from that of
Kirson.
- A putative link between inflammation,
invasion and metastasis and intra-cellular Ca2+ ion concentration has been
proposed wherein it seems the TTF modulated signal is acting rather
like Infliximab and in this
case causing Ca2+ efflux or
slowing Ca2+ entry, hence downregulating XCL2. Either way the action, brought about
by the tumour treating frequencies modulated onto the 27.12 MHz
signal is deep within the
hydrophobic part of the channel as identified by the high ICR harmonics
present. There is a distinct
breakpoint in the spread of the ICR
Harmonics for calcium across the
TTF frequency spectrum. This is
consistent with the progressive dehydration of calcium as it passes through the pore
and as documented in the literature and is also equally consistent
with a combination of both the
dielectric and angular momentum models which I have proposed.
- Unlike previous
authors which only focus on electromagnetic interaction with calcium ions
as a second messenger, it has been shown that all sorts of ion channels
and transporters can interact through ICR with their ions and/or
ligands. Indeed I have shown that the TTF’s also
effectively suppress K+ current giving the link for reduced PLP2 and
another explanation of the observed damage to the mitotic spindle.
- Simple sequential
plots of closest to integer ICR harmonic numbers for the two treatment
files yield ‘channelopathy’ like results showing break-points at places
which can be interpreted as corresponding to those of known dehydration
such as the narrow pore and selective filter.
- (5) above is
reinforced by considering the result for voltage gated proton channels
where as expected the plots are essential linear and have no sharp
breakpoints.
- Detailed cancer
specific observations of overexpressed and under expressed channels
and transporters for the two
cancers compared with the random file have been made and full explanations
in terms of the known biochemistry have been offered. Considerable success is also shown when
considering amino acids.
Further work
It is envisaged that the technique will be
developed as a new realm of contactless and drug free electronic medicine, not
only for cancer treatment but also for all kinds of other medicine where
antagonist or protagonist modification of
specific ion transit in membrane
channels may be desirable or
advantageous.
I am presently conducting further studies
based on re-analysis of Zimmerman’s data in comparison with the soliton
condensate frequencies supplied by Geesink and Meijner (G&M) in an to unify the frequencies of the ion hypothesis developed here with the
notions of Priel (2006) and Sekulic (2011) considering both solitonic ionic waves along the microtubule
axis and the transition rate of
ion channel opening and closing, local membrane conductivity, and vesicle
trafficking.
G&M have recently made such a comparison
and concluded there is no such correlation. However, I believe their argument
to fundamentally flawed as they do not seem to appreciate the crucial
dependence of the ICR frequencies on geomagnetic field.